Structural heart innovations have transformed treatment for patients who once faced open heart surgery as their only option. As minimally invasive procedures like transcatheter aortic valve replacement (TAVR) gained ground, device developers began facing new engineering challenges to solve: smaller delivery systems, improved sealing performance and reliable manufacturability at scale without compromising quality or patient outcomes and quality of life.
Every iteration brought lessons from the field. Clinical data revealed where designs could improve — with thinner materials for deliverability, three-dimensional structures for better sealing and stronger textile-to-polymer interfaces for long-term durability. This has driven the need for more specialized biomaterials that could meet these evolving demands.
Across the industry, engineers continue to ask the same critical questions:
- How can we design thinner, more flexible materials without losing strength or integrity?
- How can we translate successful prototypes into reliable, scalable production?
- How can we ensure transcatheter heart valves achieve the same long-term durability as traditional surgical valves that can last 20 years or more?
- How can we apply TAVR learnings to mitral and tricuspid therapies, where access and innovation are still limited?
- And how can we do all this while meeting increasingly complex regulatory and clinical requirements?
These are the challenges driving the next generation of structural heart innovation, and the ones that demand deep materials expertise and true partnership.
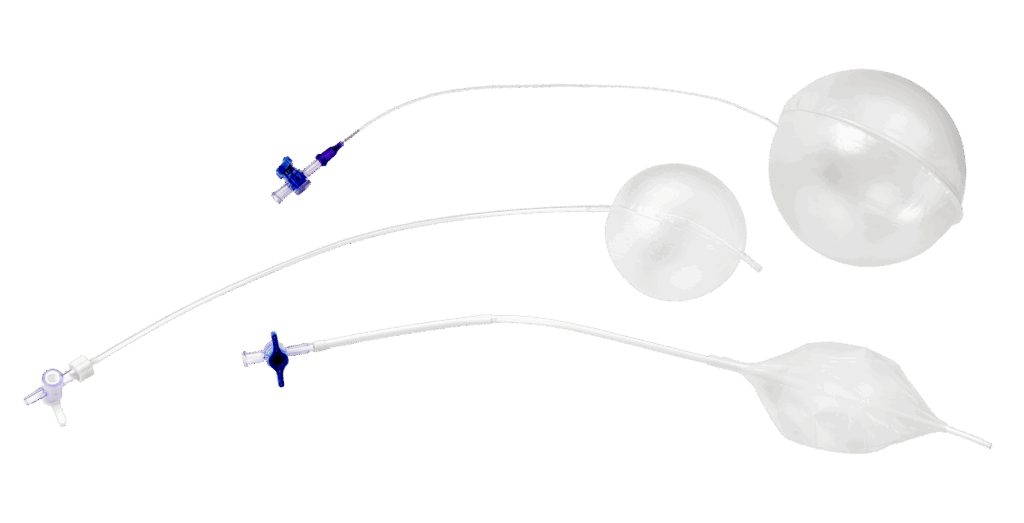
Why Expertise and Scalability Matter
Bringing a device from concept to commercialization takes more than great design. It requires materials that balance performance, manufacturability and scale. For engineers, that means anticipating production realities as early as possible.
Many companies struggle during scale-up, where designs move from R&D to production. Without the right process controls, that transition can introduce variability and risk. Experienced partners anticipate these challenges and establish scalable processes upfront, maintaining quality and consistency as volumes increase.
Traditionally, structural heart companies kept both R&D and manufacturing in-house to maintain control. Today, the ability to achieve that same level of precision and scalability through an external partner offers significant strategic value, especially as device indications expand and procedure volumes grow worldwide.
Customization Is Also Critical
As the structural heart field evolves, new performance insights continue to shape device design. Thinner, more three-dimensional fabrics have proven effective for improving sealing and conformability — and the lessons learned from early TAVR development are now informing the next frontier of innovation in aortic, mitral and tricuspid regurgitation devices.
Early-generation devices often relied on flat, low-profile materials that limited adaptability. As clinical data revealed challenges such as leakage and anatomical variation, developers began exploring more advanced textile architectures and surface treatments to enhance fit, sealing and long-term durability.
Customization has become essential as each new indication brings unique mechanical and biological demands. The ability to tailor materials to precise performance requirements while maintaining manufacturability and scalability is now a defining advantage in structural heart innovation.
Meeting these engineering and manufacturing demands requires a partner with deep materials expertise — one that understands how textiles and polymers perform from prototype to production.
How Solesis Enables Innovation in Structural Heart
To meet these challenges, innovators across the industry are partnering with Solesis — an expert in biomaterials integration that brings together the expertise of Secant Group and Polyzen under one company.
Solesis unites decades of experience in biomedical textiles, polymer films, coatings and processing technologies to help medical device companies develop the components that make life-saving structural heart technologies possible.
Within the structural heart space, Secant Group’s textiles form the foundation of many implantable devices and delivery systems, offering both off-the-shelf options for easy prototyping and fully customized solutions to meet performance and manufacturability needs. Polyzen complements this with polymer expertise, developing films, coatings and delivery system components that enable thinner profiles, enhanced sealing and next-generation device designs.
Together, these Solesis companies provide integrated biomaterial solutions that support every stage of innovation, from concept through commercialization.



Accelerating Early Adoption With Off-the-Shelf Fabrics
When structural heart procedures first emerged, device developers needed validated materials that could support proof-of-concept work and shorten development timelines. Solesis anticipated this need with a portfolio of low-profile, off-the-shelf fabrics that gave engineers reliable, ready-to-use options for early prototyping.
These materials could be used immediately or refined for specific systems, giving developers the flexibility to advance their programs efficiently. Many of those early fabrics went on to become part of fully approved devices that are still in use.
Today, Solesis provides a range of customizable textile pathways — from validated, off-the-shelf fabrics to fully customized solutions tailored to specific device requirements. This gives developers a scalable starting point that can evolve with their designs, helping them:
- Innovate quickly and cost-effectively with ready-to-use fabrics
- Save costs by prototyping internally with validated materials
- Identify the right fabric for their performance needs
- Avoid biocompatibility issues early in development
- Access expert textile engineering support throughout design
Partnering Across the Innovation Spectrum
Not every partner arrives with the same needs. Some bring precise specifications, while others begin with a sketch and a problem statement. From the earliest stages of the structural heart field, Solesis has worked alongside innovators to refine ideas, solve design and manufacturing challenges, and help their mission to bring safe, effective devices to market.
For larger original equipment manufacturers (OEMs), Solesis’ broad platform and technical expertise provide assurance. Its materials are used across a significant portion of structural heart devices, giving developers confidence in both the technology platforms and their performance in real-world applications.
Solesis also continues to collaborate with startups and small-to-medium-sized enterprises, helping them advance novel concepts and, in some cases, position for acquisition by larger players.
This full-spectrum partnership model keeps Solesis closely aligned with innovation at every level of the industry.
Preparing for Expanding Indications and Rising Demand
The future of the structural heart device industry will be defined by growth and driven by the expanding body of clinical data supporting the efficacy of these interventions. Procedure volumes continue to climb, and approvals are expanding into new indications, including mitral, tricuspid, aortic and valve-in-valve interventions for regurgitation, stenosis and other structural valve diseases. These advancements are also broadening patient eligibility, making less invasive options available to individuals who previously had no viable or limited treatment or were considered too high-risk for open surgery.
As indications expand and technologies advance, the need for scalable, high-quality biomaterials continues to rise. Solesis invests in multiple manufacturing facilities to support customers as they scale, ensuring consistent quality and supply as demand accelerates.
Meeting these demands requires both materials innovation and assembly efficiency. Looking further ahead, new approaches such as advanced coating techniques and electrospun materials may enable thinner device profiles and more conformable sealing solutions.
Leadership Through Innovation and Delivery
From the first generation of structural heart devices to today’s advanced solutions, Solesis has been a trusted partner at every stage, anticipating market needs, responding to clinical data, enabling scalability and supporting innovators of every size.
As the next generation of structural heart devices takes shape, Solesis remains deeply involved in helping innovators bring safer, more effective therapies to market — through advanced biomaterials, scalable manufacturing and collaboration at every stage of the process.
